All families should be able to make informed decisions about infant and young child feeding based on objective factual information free from confusing and exploitative marketing and commercial interests.
What are the main elements of the WHO Code?
- Companies that manufacture BMS should have no direct or indirect contact with pregnant women and mothers of babies and young children. This includes any type of advertising, discount promotions, special displays, samples, gifts and sponsorship of parenting clubs.
- Health professionals do not have a role in promoting breastmilk substitutes, or bottles and teats (this does not prevent individual education on safe use of formula).
- Standards of ethical behaviour are set for industry employees, including prohibiting the calculation of bonuses based on sales of formula milk, bottles or teats.
- Clear labelling of products are required and pictures or text that idealise the use of breastmilk substitutes are prohibited. (Read below for more detail).
What is a breastmilk substitute?
Breastmilk substitutes include any milks (or products that could be used to replace milk such as fortified soy milk), which are specifically marketed for feeding children up to the age of 3 years, including follow-on formula and growing-up milks (toddler milks).
The WHO Code also seeks to prevent inappropriate promotion of foods and drinks as being suitable for feeding a baby during the first 6 months of life when exclusive breastfeeding is recommended.
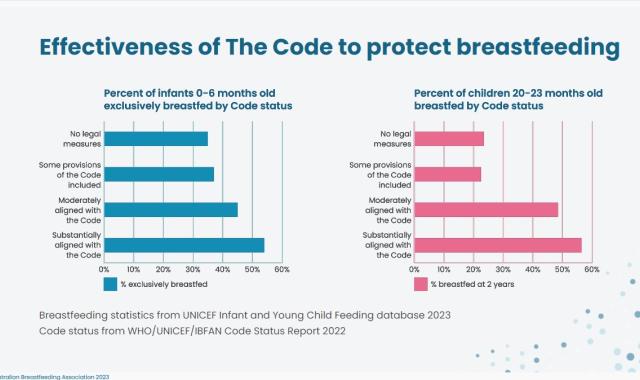
The International Code of Marketing of Breast-milk Substitutes (WHO Code) in detail
The WHO Code primarily covers breastmilk substitutes, feeding bottles and teats.
Breastmilk substitutes include any milks (or products that could be used to replace milk such of fortified soy milk), which are specifically marketed for feeding children up to the age of 3 years, including follow-up formula and growing-up milks.
The WHO Code also seeks to prevent inappropriate promotion of foods and drinks as being suitable for feeding a baby during the first 6 months of life when exclusive breastfeeding is recommended.
The Code states that governments are responsible for ensuring objective and consistent information is provided on infant and young child feeding, both to families and others involved in infant and young child feeding.
Materials should clearly state the importance of breastfeeding as well as the risks and costs of formula feeding.
Limits are also placed on donations of information and education materials by manufacturers of breastmilk substitutes, feeding bottles and teats.
The WHO Code explicitly states that breastmilk substitutes, feeding bottles and teats should be available but not promoted. This includes any type of advertising, discount promotions, special displays, samples, gifts and sponsorship of parenting clubs.
Companies should have no direct or indirect contact with pregnant women and mothers of babies and young children.
The WHO Code covers all promotion by manufacturers, distributors and retailers (e.g. supermarkets and pharmacies).
The WHO Code prohibits promotion of breastmilk substitutes, feeding bottles and teats through healthcare services. Health services should not have displays, posters or other promotions for breastmilk substitutes, feeding bottles and teats. Manufacturers of breastmilk substitutes, feeding bottles and teats should not provide parent education through health services.
Information and education on the proper use of infant formula should only be provided to those who require it – there should be no general education classes provided on infant formula.
The WHO Code prohibits financial inducements given to health workers to explicitly promote products covered by the Code. The Code also prohibits the distribution of samples by health workers to the general public.
The WHO Code prohibits sponsorship and other financial or in-kind inducements which may present a conflict of interest for health workers.
The International Code of Marketing of Breast-milk Substitutes: Frequently asked questions on the roles and responsibilities of health workers contains information on health workers' obligations to the Code.
The WHO Code prohibits the calculation of bonuses from sales of breastmilk substitutes, feeding bottles and teats. The Code also prohibits employees of manufacturers of breastmilk substitutes, feeding bottles and teats from providing education to pregnant women or mothers.
The WHO Code restricts labelling to factual information only. It prohibits nutrition or health claims and images which may idealise the product.
The WHO Code also requires that all breastmilk substitute labels include a statement on the superiority of breastfeeding, that the product should only be used on health advice, and instructions on appropriate preparation.
Warnings outlining the risks of inappropriate preparation and the risk of breastmilk substitutes containing pathogens are also required.
The WHO Code acknowledges the quality of products is vital to the health of infants and products should meet standards set by the Codex Alimentarius Commission.
The WHO Code outlines that it is the responsibility of governments to take action to ensure the WHO Code is implemented through suitable measures and applies to all manufacturers and distributors.
The WHO Code also outlines the responsibilities of others in upholding the Code, including non-governmental organisations, professional groups and individuals.

Report a breach of the WHO Code in Australia

The WHO Code FAQs

Report: Impact of Formula Milk Marketing
